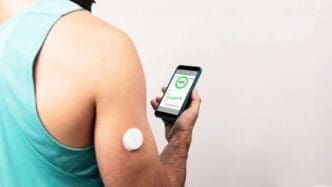
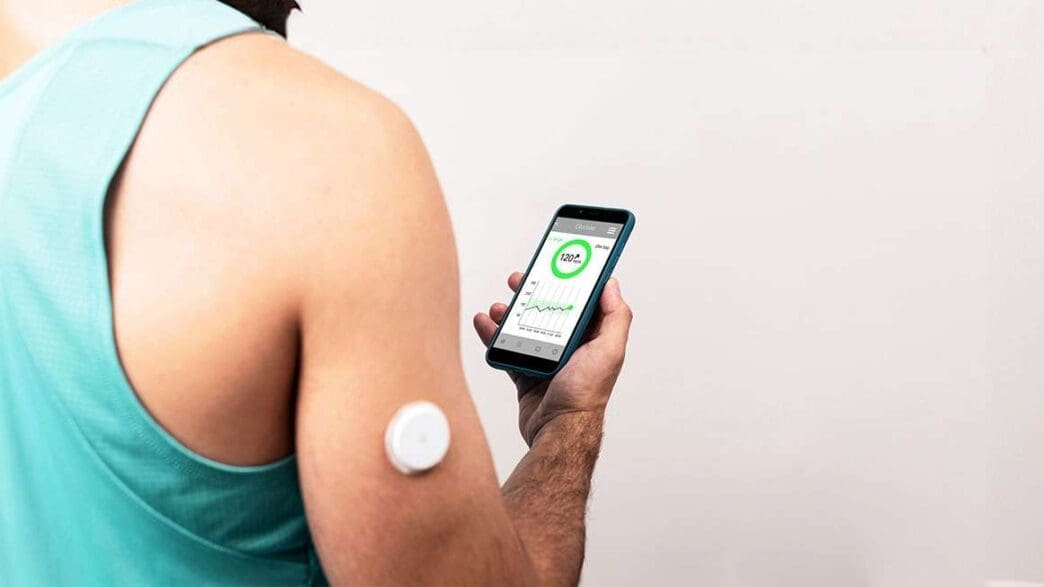
The U.S. Food and Drug Administration (FDA) has taken a groundbreaking step by approving the first over-the-counter continuous glucose monitors (CGMs) for sale. This decision is seen as a significant move toward greater health accessibility for all.
CGMs have long served as indispensable tools for those managing diabetes. These wearable devices offer real-time monitoring of blood sugar levels, allowing individuals to track changes without the hassle of traditional finger-prick methods. However, their utility extends beyond diabetes management. Even individuals without a diabetes diagnosis can benefit from these devices, particularly if they have high blood sugar levels that don’t yet meet the clinical threshold for diabetes.
Previously, individuals keen on understanding their blood sugar patterns encountered barriers due to prescription requirements. As CGMs were exclusive to diabetics with a doctor’s prescription, many interested in these gadgets had to seek doctors willing to prescribe them on an off-label basis, often dealing with cumbersome processes and high costs. But thanks to the FDA’s recent approval, individuals can now purchase these devices directly, empowering them with valuable health insights.
Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, emphasizes this move as an important step toward health equity, allowing broader access to critical health information. He notes that increasing access to these devices can help people manage their health proactively, even without regular doctor visits or health insurance.
The variety of glucose monitors available over the counter has expanded significantly. Dexcom, for example, now offers the Stelo Glucose Biosensor System, which is available for both diabetics not using insulin and individuals without diabetes but interested in understanding the impact of diet and exercise on their blood sugar. Similarly, Abbott’s Libre Rio targets Type 2 diabetics not using insulin, while their Lingo sensor serves non-diabetics seeking to monitor their glucose levels. These advancements bring CGMs within reach for a wider audience.
Although some debate persists in the health community regarding the use of CGMs by non-diabetics, these devices provide users with critical data points that can influence lifestyle and dietary decisions. The potential to personally monitor and adjust one’s health trajectory places control firmly in the hands of consumers, rather than strictly within the confines of medical professionals and prescriptions.
In terms of cost, these over-the-counter options offer a more budget-friendly alternative. While subscription services like Signos offer CGMs at a premium, direct purchases have made them more affordable. Consumers can now access the Stelo system at $99 per month, or $89 with a subscription, compared to the higher costs associated with medical consultations and traditional monitoring methods. Single-use biosensors like Abbott’s Lingo also offer cost-effective solutions at $49 for a two-week use or $249 for a 12-week supply.
The FDA’s approval of over-the-counter CGMs represents a shift towards consumer empowerment in health management. By removing prescription barriers, individuals gain the ability to actively manage and understand their health metrics, leading to more informed health and lifestyle choices. This regulatory change not only democratizes access to health technology but also sets a precedent for future innovations in personal health monitoring.
Source: Yahoo