The Food and Drug Administration (FDA) has issued a nationwide recall of a specific lot of eye drops due to a potential fungal contamination risk, posing a threat to vision health.
Alcon Laboratories, a Texas-based company, has voluntarily recalled a single lot of its ‘Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go’. This decision followed a consumer’s report of discovering foreign material inside a sealed vial, which was identified as being fungal in nature.
Fungal contamination in eye products can lead to eye infections, as stated by the FDA. These infections could threaten vision, and in very rare situations, they may even pose a life-threatening risk, particularly to immunocompromised individuals.
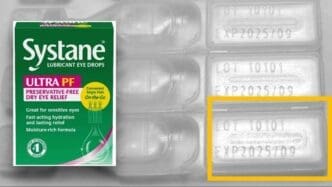
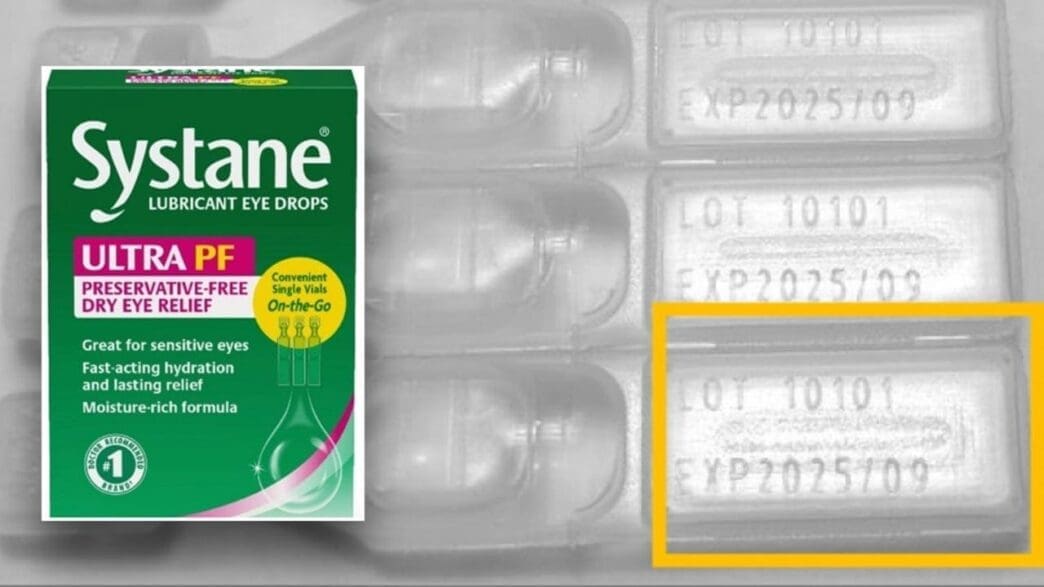
The recall is specific to the Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count, limited to lot number 10101 with an expiration date of September 2025. The product packaging is identifiable by its green and pink carton, which clearly displays the ‘Systane’ and ‘ULTRA PF’ branding, along with the ’25 vials’ package indication.
Furthermore, the affected lot was distributed nationwide across various retail and online platforms. The FDA strongly advises consumers who possess these eye drops to cease usage immediately and return the products to their purchase point for a refund or replacement. Similarly, retailers and distributors are urged to discard any remaining stock of this potentially contaminated batch.
Alcon Laboratories is actively reaching out to its distributors and customers through letters, emails, and phone calls to arrange for the replacement of recalled products. This recall is part of a broader concern over eye product safety, following earlier incidents this year where eye ointments sold at major retailers like CVS and Walmart were recalled due to sterility issues at manufacturing facilities.
This series of recalls highlights ongoing challenges in ensuring the sterility and safety of eye care products, a crucial aspect since the effects on vision can be significant. Previous recalls by other companies such as Kilitch Healthcare India Limited concerned bacterial contamination, underscoring the need for stringent quality control measures in the production of these products.
The FDA continues to monitor the situation, ensuring that all potentially contaminated products are removed from circulation to protect public health. Consumers should remain vigilant and report any adverse reactions or concerns to healthcare providers.
Source: Fox13news