The risk of Alzheimer’s disease, especially among those with genetic predispositions, is a topic of intense study. While genetics can dictate a high likelihood of developing Alzheimer’s, research suggests other factors might play a role in mitigating these risks. A recent case presents a fascinating exception, prompting discussions about potential protective elements within the brain.
Scientists are diving deeper into understanding this anomaly to unravel potential preventive strategies. The examination of individuals who show unexpected resilience to Alzheimer’s offers clues that could reshape our approach to treatment. This article investigates the underlying mechanisms of such resistance and what they mean for future therapeutic developments.
Case Study: A Remarkable Exception
In 2011, researchers encountered Doug Whitney, a 75-year-old man with a genetic marker linked to early-onset Alzheimer’s. Despite this genetic burden, Whitney has remained symptom-free, challenging expectations. This case stands out as it contradicts what genetics would predict about his health. Whitney’s experience is part of a broader study aiming to understand the unpredictability of Alzheimer’s development.
Dr. Jorge J. Llibre-Guerra, involved in the study, highlights the potential of unexpected genetic factors. The case not only shines a light on this particular form of Alzheimer’s but enriches our broader understanding. Such studies might pave the way for more effective management strategies applicable to the general population, offering a unique research opportunity into disease prevention.
Tau Protein’s Role in Alzheimer’s
Typically, people carrying the PSEN2 mutation develop symptoms in their 50s. Whitney’s lack of cognitive decline led researchers to initially doubt he carried the gene. His status as an ‘escapee’ was confirmed once they verified his genetic background. His resilience sparked interest in what biologically shields him.
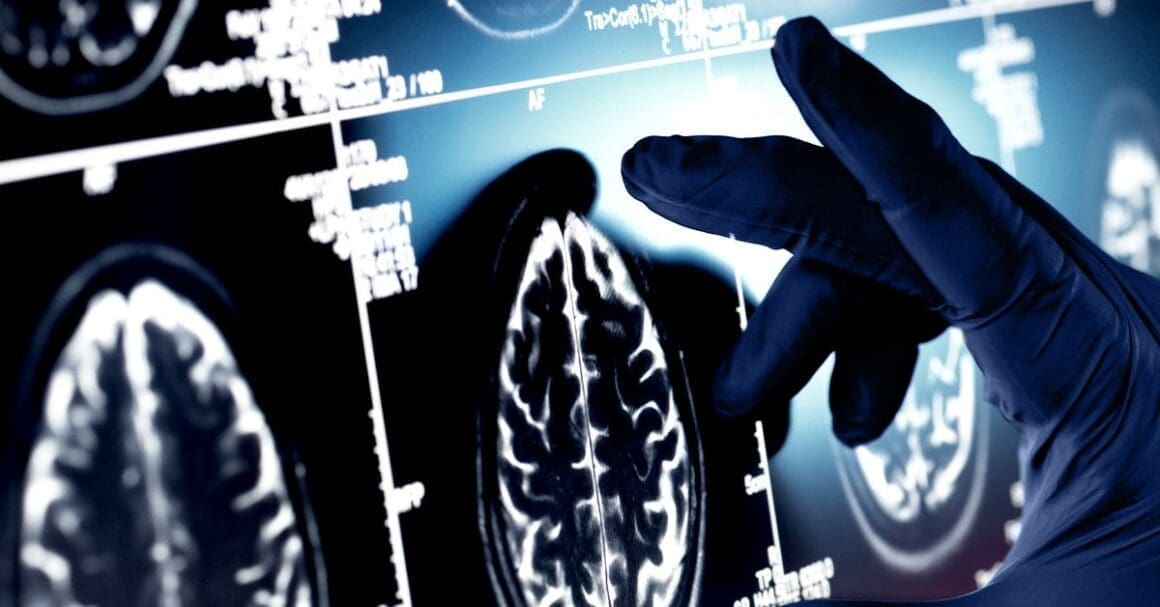
Brain scans revealed Whitney’s brain had amyloid, commonly linked with Alzheimer’s, yet minimal tau protein build-up. Tau is considered a critical factor in Alzheimer’s progression. This atypical protein distribution might suggest existing protective mechanisms, preventing the full development of Alzheimer’s symptoms.
Searching for Mechanisms of Resistance
At this juncture, there’s no clear explanation for Whitney’s resistance to Alzheimer’s.
Dr. Llibre-Guerra hypothesizes it may involve multiple factors, including unique genetic expressions and environmental influences. Factors like these could induce protective brain responses, contributing to his resilience.
The study’s insights could direct future research towards therapies that mimic or amplify these protective effects to benefit a wider audience.
Challenges and Skepticism in Research
Some experts express doubts about the findings. Dr. Clifford Segil questions the study’s conclusions about tau, particularly its minimal presence.
According to Dr. Segil, there’s an expectation that tau would contribute to symptoms if present, especially in certain brain regions. This anomaly raises questions about current scientific understanding of tau’s impact on Alzheimer’s.
The ambiguity surrounding tau’s role might redirect research towards examining its effects in other disorders, like Parkinsonism, where its presence is more definitive.
Genetic Insights and Alzheimer’s Prevention
The presence of protective genetic variants in individuals like Whitney opens new avenues for Alzheimer’s research.
Dr. Jasmin Dao notes the importance of identifying neuroprotective genetic markers that could alter disease progression. These findings underscore the potential for preventive strategies, particularly in genetic Alzheimer’s cases.
By focusing on the genetics behind resistance to Alzheimer’s, researchers might unlock novel treatments that could slow or prevent cognitive decline in vulnerable populations.
Current therapies focus on early detection and slowing disease progression.
Insights from individuals with resistant genetic profiles could inform new approaches to both early-onset and late-onset Alzheimer’s, facilitating more effective prevention and management.
Broader Implications for Neurological Research
The study of Whitney’s genetic anomaly is encouraging further exploration into neurological resilience.
By understanding how genetic factors can protect against cognitive decline, the field of neurology may gain new therapeutic targets.
Dr. Dao’s insights suggest novel treatment possibilities that extend beyond Alzheimer’s, potentially benefiting various neurodegenerative disorders.
Whitney’s case might reveal common pathways that safeguard against brain disorders.
Neurologists believe these genetic pathways, if harnessed, could revolutionize the way we treat age-related cognitive diseases.
Future Directions in Alzheimer’s Research
Investigating genetic resistance can illuminate new strategies for combating Alzheimer’s.
As researchers dig into mechanisms that might curb the spread of tau pathology, the hope is to develop actionable therapies.
These therapies would aim to mimic the natural defenses observed in individuals like Whitney.
Such research offers promise not only for Alzheimer’s but could also affect treatments for related diseases.
The potential to identify protective factors is an exciting frontier, offering hope for those genetically predisposed to Alzheimer’s.
Current understanding of tau’s role might be reevaluated, with a longer view towards prevention and treatment.
Researchers are focusing on how these variants restrict tau’s advancement, considering applications across neurodegenerative disorders.
Dr. Llibre-Guerra and his team envision translating these insights to practical, preventative interventions.
Their work emphasizes the possibility of transformative breakthroughs in Alzheimer’s care, rooted in genetic and proteomic study.
Such developments have the potential to reshape therapeutic landscapes, offering better outcomes for an aging population.
As scientists work to decode resilience to Alzheimer’s, this research may herald transformative therapeutic strategies. By leveraging genetic insights, there’s hope not only for prevention but also for improved treatment of those at risk.