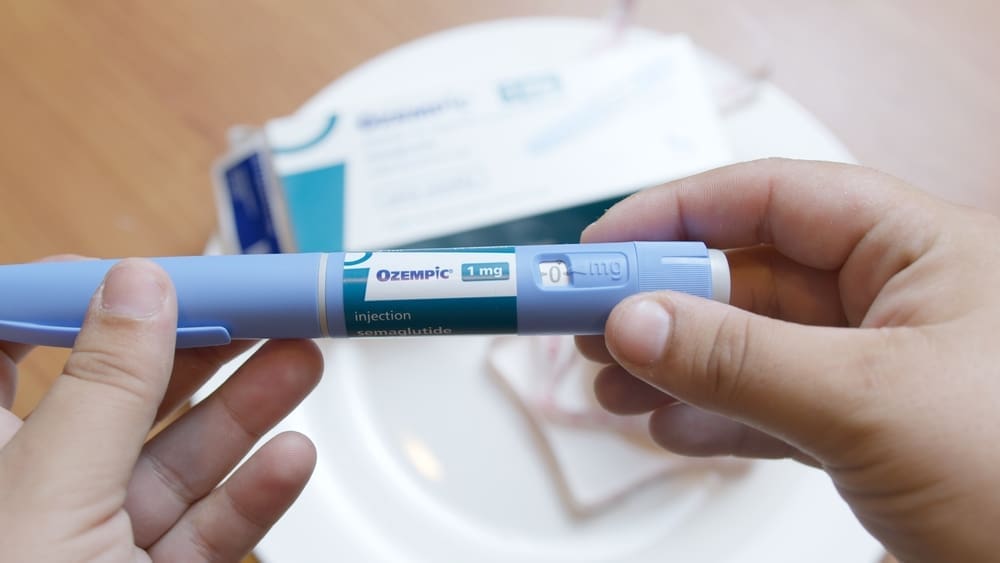
The Food and Drug Administration (FDA) may soon prohibit compounded versions of Ozempic and Wegovy following the stabilization of their supply. These medications, which contain semaglutide—a glucagon-like peptide 1 (GLP-1) receptor agonist—were added to the FDA’s drug shortage list in August 2022 due to increased demand, particularly for off-label weight loss uses. With the shortage resolved, the FDA has signaled that compounding pharmacies must cease production of these versions within 60 to 90 days or face enforcement actions.
The Outsourcing Facilities Association (OFA), representing these pharmacies, has challenged the FDA’s decision with a federal lawsuit, leaving the future of compounded semaglutide uncertain for many users. The FDA allows compounded drugs only when there is a shortage or if a patient’s specific needs cannot be met by the FDA-approved drug. These compounded versions have gained popularity in the U.S., especially for weight loss, as they are significantly cheaper than the branded medications, which are not typically covered by insurance for non-FDA-approved uses.
Jennifer Carter-Johnson, a law expert in food and drug regulation, notes that compounded versions can cost just 10% to 15% of the branded drugs. However, compounded drugs do not undergo the same safety or efficacy testing as FDA-approved medications. Despite this, demand has surged, partly due to the high cost of branded versions of semaglutide medications.
The FDA has postponed enforcing its ban on compounded semaglutide to avoid disrupting patient care, giving state-licensed pharmacies until April 22 and larger outsourcing facilities until May 22 to comply. The OFA argues that allowing compounded versions provides necessary access, especially given ongoing supply concerns, and claims the FDA relied solely on data from branded companies rather than broader sources.
A similar legal challenge arose with the drug tirzepatide, where the court ruled against the OFA, eventually leading the FDA to implement a ban. This could set a precedent for the semaglutide situation, as Carter-Johnson explains. The current legal proceedings will determine the timeline for any potential ban.
In addition to supply issues, the FDA cites safety concerns for the ban, having received over 455 reports of adverse events linked to compounded semaglutide. These incidents, ranging from dosing errors to unapproved dosing, highlight risks associated with compounded drugs.
Experts like Carter-Johnson warn that a ban might drive patients to unsafe alternatives, such as counterfeit drugs from unauthorized sources. They urge patients to adhere to FDA-approved medications and explore alternative weight loss solutions if affordable options are not available.
The Human Angle: Navigating the Impact on Individuals and Communities
- Cost Implications: Consumers who rely on compounded versions for affordability may face significant financial burdens if they need to switch to more expensive branded drugs.
- Access to Medications: With compounded versions potentially banned, patients might struggle to access necessary treatments, especially if insurance coverage remains limited.
- Safety Concerns: The shift away from compounded drugs may push people toward unregulated or counterfeit options, posing serious health risks.
- Legal and Regulatory Impacts: The outcome of the FDA’s legal challenges could shape future policies on drug compounding and influence pharmaceutical industry practices.
- Community Health Resources: Local pharmacies offering compounded medications may experience economic impacts, potentially affecting community health service availability.
- Public Awareness: This issue highlights the need for increased public understanding of drug sourcing, safety, and regulatory processes, fostering more informed healthcare choices.